Challenges
The inclusion criteria of this global study were challenging and necessitated significant resource to screen patients. Consequently, very few patients were being identified, ongoing pre-screening activity was limited and minimal screening visits were being conducted.
A clear and well defined recruitment strategy had not been developed, resulting in poor engagement from site staff and a negative perception of the study.
CRA recruitment support and funding of a Research nurse by the Sponsor had made little impact.
Solutions
O4 took stock of the study landscape upon joining the project, before developing an overarching study action plan.
Deployment of O4 TransformTM enabled the recruitment strategy to be optimised, including the provision of a range of customised recruitment support materials. Off-site management support was provided and the O4 TransformTM strategy employed to re-energise the study and increase communication within sites and across study teams.
Additionally, O4 Research provided dedicated, custom managed on-site Clinical Research Coordinator support across the majority of sites to further enhance performance.
Impact
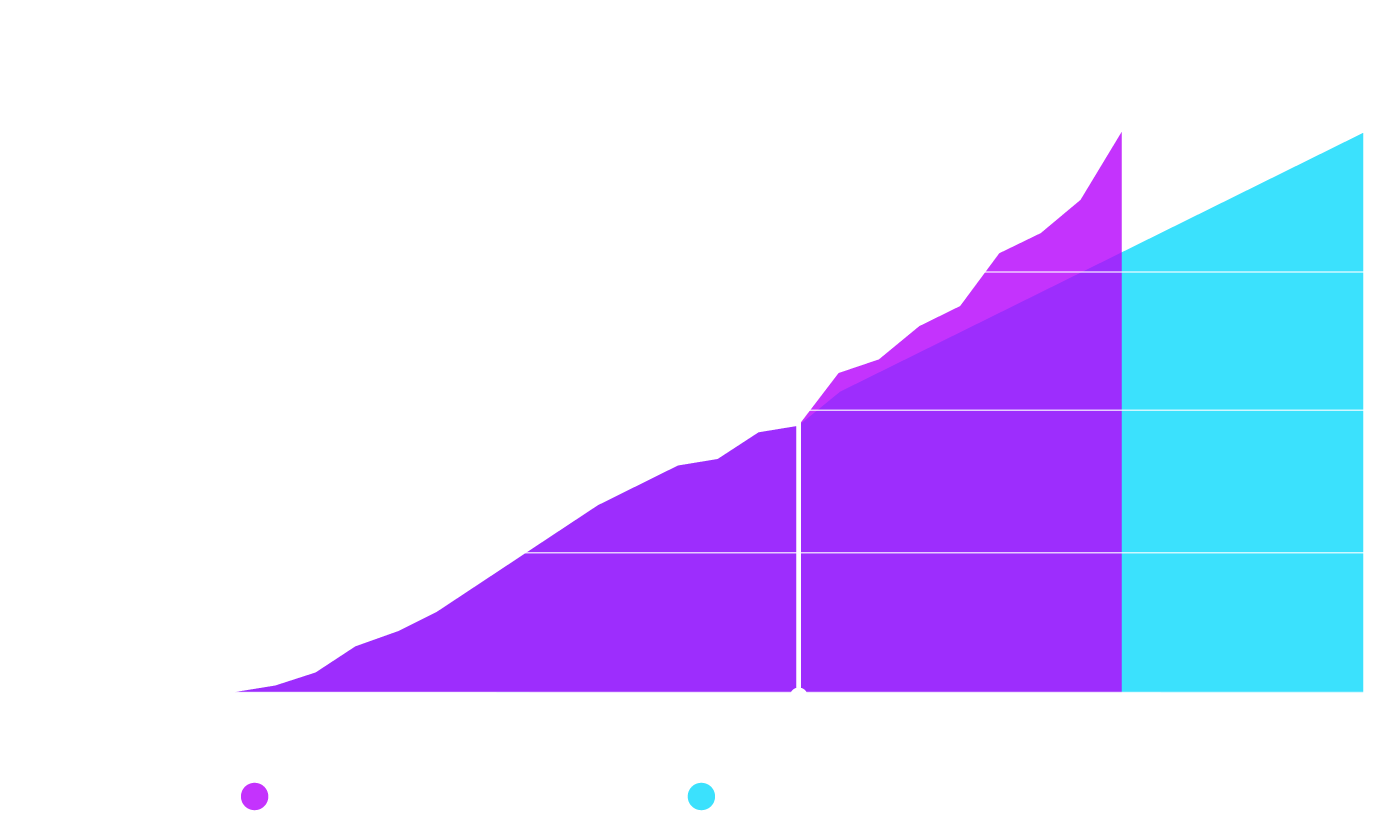
The dramatic uplift in subject enrolment reduced the projected recruitment period by 6 months. In fact, O4 enabled both the screening and enrolment of more patients in 8 months than was managed in the 16 months prior to O4 involvement.