Challenges
Recruitment for this multi-centre study was protracted, with some sites not having recruited a single patient during a 12 month period whereas recruitment had stalled at other sites. Consequently, sites had become disinterested and there was a general negative perception of the study.
Solutions
O4 was contracted to take over site selection, site management, monitoring and local project management, to effectively ‘rescue’ the study and achieve the recruitment target.
–
O4 TransformTM was utilised to re-energise the study, taking both a strategic and patient centric approach.
–
O4 EngageTM was applied to ensure effective communication across the study team and create a unique vibrant identity for the study, making it more attractive for potential patients and raising the study profile within the research sites. Finally, O4 AccelerateTM techniques were employed to fast-track patient recruitment including the involvement of O4s community pharmacy network.
Impact
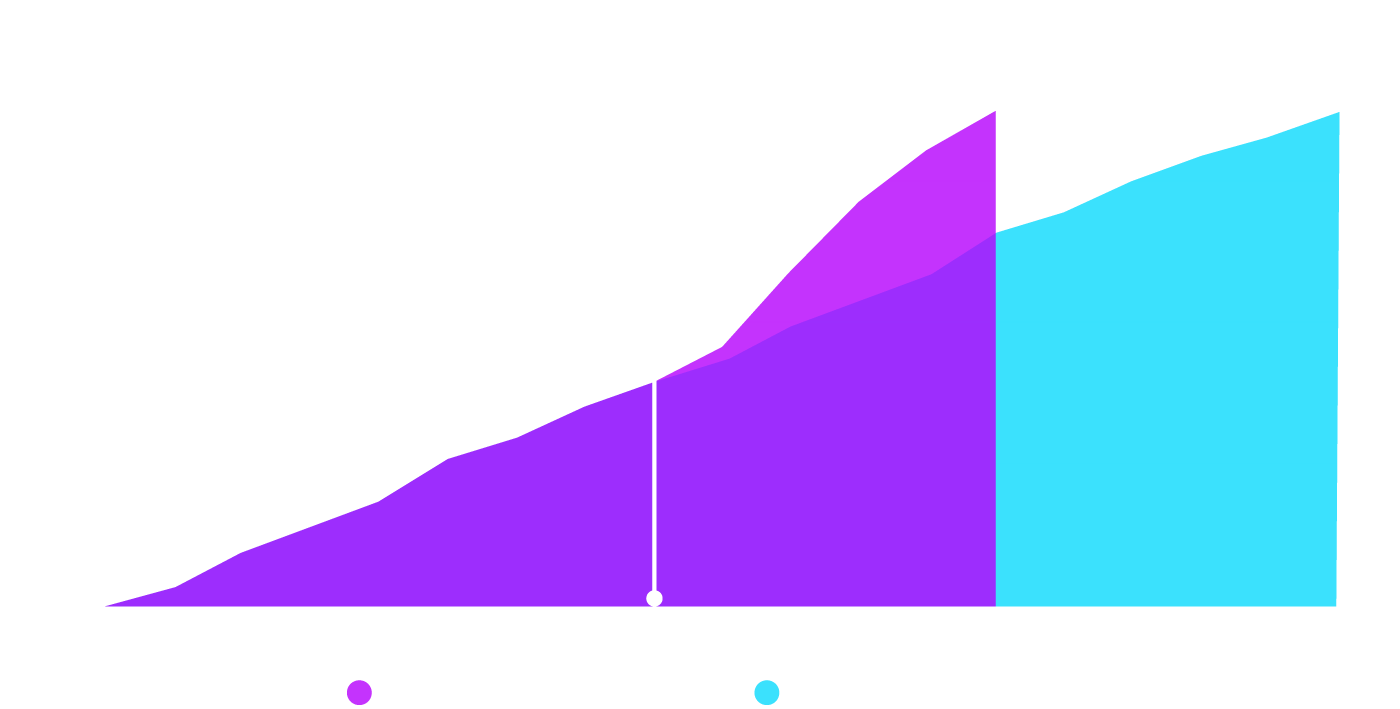
The O4 TransformTM initiative had a major impact on the level of engagement and patient recruitment, reducing the anticipated recruitment period by 15 months.